Abstract
Background: Between April 2012 and July 2018, adult patients aged 25-65 years with newly diagnosed T-cell acute lymphoblastic leukaemia (T-ALL) in 48 UK centres were randomised at trial entry to receive standard of care (SOC) ALL induction phases I and II chemotherapy with or without the addition of single-agent nelarabine.
Nelarabine 1.5 g/m 2 was given on days 1, 3 and 5 following the second phase of induction. The trial had 86% power to detect a 25% improvement (50-75%) in 3-year event free survival (hazard ratio [HR]=0.42, events: relapse and death) and a two-sided alpha 0.05.
Early T-cell progenitors express high levels of SAMHD1, a gene recently shown to mediate resistance to nelarabine(Rothenburger et al, Commun Biol, 2020). We retrospectively assessed diagnostic samples for their level of differentiation arrest through analysis of the TCRg locus. Here, the most immature cases are detected through absence of biallelic TCRg deletion (ABD)(Farah et al, Haematologica, 2018). Results were correlated with survival outcomes.
Results: 144 eligible patients were recruited, of whom 75 were randomised to SOC and 69 to SOC plus nelarabine (SOC+N). Baseline characteristics were balanced for factors including age (median age 38 years), presenting white cell count and performance status with a slightly greater proportion with cytogenetic high-risk factors in the SOC arm (8.5% vs 20%).The complete remission (CR) rate following induction phase I and II was 90.7% in the SOC arm and 87.0% in the SOC+N arm.
The rate of severe adverse events and non-relapse mortality did not differ between the arms. There was no increase in grade 3-4 neurotoxicity reported in patients who received nelarabine with 6 events (9.0%) reported in the SOC arm during phase II induction and 7 events (11.9%) in the SOC+N arm. There were no deaths reported from neurotoxicity in either arm.
Of those randomised at diagnosis to receive SOC+N, 48 (71.0%) actually received the agent, as those with persistent therapy-related toxicities were excluded. Forty-three patients (62.3%) received all 3 planned doses. Analysis is by intention-to-treat.
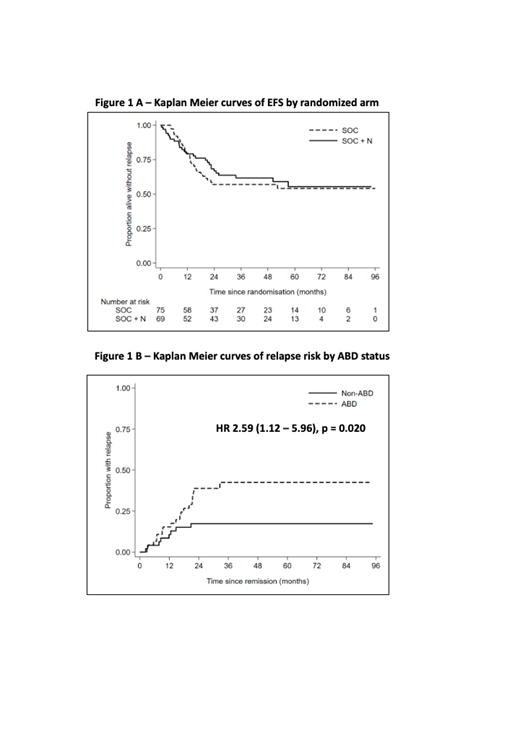
At a median follow up of 51.9 months, 3-year EFS was 57.0% (95% confidence interval [CI] 44.7%-67.5%) in the SOC arm vs. 61.7% (48.7-72.4) in the SOC+N arm; HR 0.88 (0.52-1.46; p=0.61) (fig 1A). Overall survival (OS) at 3 years was 61.5% (49-71.8) SOC vs 65.7% (52.6-76) SOC+N; HR 0.91 (0.53-1.56; p=0.73). 133 patients achieved CR and were assessable for relapse; relapse rates (RR) at 3 years were similar, 29.1% (19.7-41.9) in the SOC arm vs 28.0% (18.4-41.3) in the SOC+N arm; HR 0.97 (0.48-1.92; p=0.91). An "as-treated" analysis did not change conclusions.
Outcomes were improved compared to our prior trial, UKALL 12 despite a higher median age in UKALL 14; 5-year OS and RR 58.0% (48.3 - 66.5) and 28.7% (21.6 - 37.6) vs 48.0% (42- 53%) and 42.0% (36-47%).
ABD status at diagnosis was examined in 108 patients. Of these, 48 were non-ABD, 46 ABD and 14 indeterminate. Patients with ABD were less likely to be MRD negative post-induction (26.0 vs 58.0%, p=0.001) and had a significantly higher relapse rate at 3 years compared to patients without ABD (fig 1B), 42.5% (29.0 - 59.2 ) vs 17.4% (9.1 - 31.8 ); HR 2.59 (1.12 - 5.96, p=0.02). This result held when adjusted for other baseline risk factors. The proportion of patients receiving allogeneic transplantation was similar (58.8% non-ABD/56.9% ABD, p = 0.84). The interaction with treatment arm was not significant (p=0.46).
Clinical implications: Three doses of nelarabine added to standard chemotherapy in the treatment of adult T-cell ALL patients was well tolerated. However, there was no demonstrable EFS or OS benefit seen with the addition of nelarabine. Survival outcomes for patients with T-cell ALL in the UKALL 14 trial were improved compared to UKALL12 despite the older age of patients in UKALL14 suggesting that other protocol factors had a positive effect on outcomes. The paediatric COG AALL0434 study showed a DFS benefit for nelarabine given as six 5-day courses. It is possible that too few doses were given in UKALL14 to show benefit.
We identified ABD as an independent negative prognostic factor within this cohort of adult patients. Patients with T ALL and ABD had over twice the risk of relapse, suggesting that they should be considered high-risk in future studies. We were not able to demonstrate a benefit of nelarabine according to ABD status.
Clifton-Hadley: Millennium pharmaceutics inc.: Other: The haematology team at the CTC has received funding (which in part pays staff salary) to Sponsor and coordinate clinical trials.; Pfizer: Other: The haematology team at the CTC has received funding (which in part pays staff salary) to Sponsor and coordinate clinical trials.; Janssen-Cilag: Other: The haematology team at the CTC has received funding (which in part pays staff salary) to Sponsor and coordinate clinical trials.; Merck Sharp and Dohme: Other: The haematology team at the CTC has received funding (which in part pays staff salary) to Sponsor and coordinate clinical trials.; Amgen: Other: The haematology team at the CTC has received funding (which in part pays staff salary) to Sponsor and coordinate clinical trials.; Celgene: Other: The haematology team at the CTC has received funding (which in part pays staff salary) to Sponsor and coordinate clinical trials.; Bristol-Myers Squibb Pharmaceuticals Ltd..: Other: The haematology team at the CTC has received funding (which in part pays staff salary) to Sponsor and coordinate clinical trials.. Mansour: Janssen: Consultancy; Astellas: Consultancy, Honoraria. Fielding: servier: Research Funding; Novartis: Consultancy; Amgen: Consultancy.
Nelarabine for denovo ALL